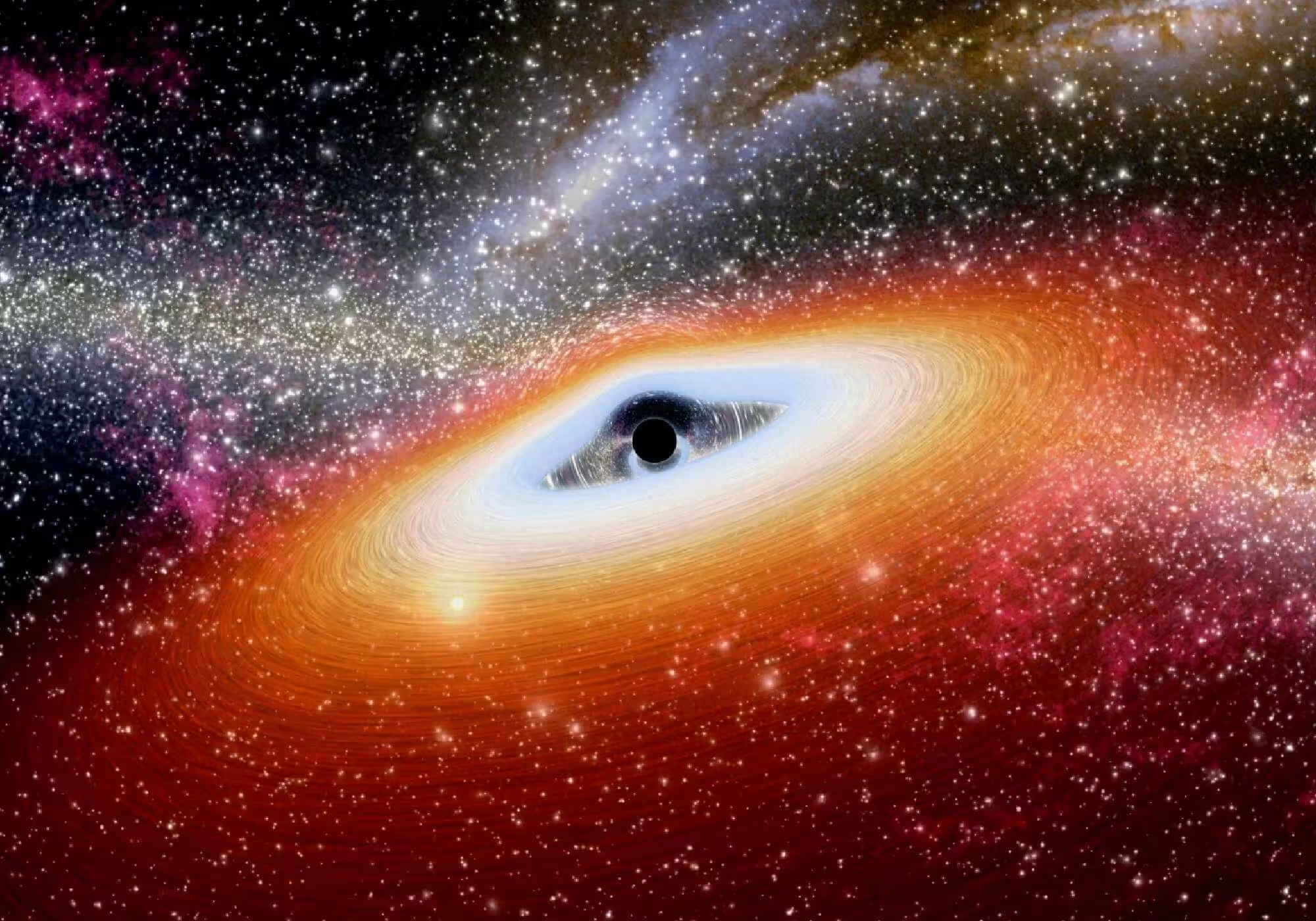
Scientific investigations show that dark matter provides essential insights into the primary mysteries present in outer space. An aerial dance occurs when invisible forces use black holes as they move toward a magnificent cosmic collision. Dark matter potentially acts as the concealed power that draws huge black holes toward their meeting points when they approach each other. Scientists consider dark matter to be the most mysterious phenomenon in the entirety of space. Technological instruments are unable to detect dark matter because it does not emit light, even though researchers understand its gravitational effects on visible objects. Scientists detect dark matter’s existence through observations, yet it remains invisible to all detection methods because it represents 27% of everything in existence. Dark matter potentially assists the merger of black holes despite their limitations, known as the Final Parsec Problem. This article examines how dark matter and black holes possibly combine to resolve the existing mystery. The Final Parsec Problem describes the problem black holes with cosmic dimensions have in forming proximity with their counterparts. We can detect the gravitational waves because black holes produce them while they approach proximity for collision. Waves detected from space prove that two black hole are uniting. The black holes maintain complete silence beyond what scientists consider the last parsec. They can’t get close enough. For numerous years, scientists have strived to resolve this matter. The knowledge of black hole comprehension relies on successfully solving this problem. Understanding galaxy formation requires this knowledge to be established. Through its study, we unveil information about the construction of the universe. Background The cosmic universe requires dark matter to function as an unseen binding agent. Scientists can detect dark matter only through its gravitational influence on observed cosmic substances, despite its ability to remain invisible to standard detection methods. The discovery of dark matter began when Fritz Zwicky found galaxies in clusters moving abnormally fast, thus indicating unobservable mass existed during the 1930s. After its initial discovery, dark matter established itself as one of the most mysterious elements, which continues to defy scientists today. Researchers currently support ideas that dark matter exists as WIMPs (Weakly Interacting Massive Particles) and axions, although no experimental team has identified either of these particles directly. The study of dark matter properties happens through a combination of gravitational lensing and cosmic microwave background radiation, since scientists cannot detect it directly. Galaxies, together with galaxy clusters, reach their shapes through the elusive gravitational impact of these vanishing particles. The structures of galaxies collapse because dark matter provides an amount of mass that averts their dissolution. Scientists continue researching dark matter to identify its composition precisely, but they have no doubt about its cosmic impacts because of the evidence. Researchers have identified the potential for dark matter to modify supermassive black hole interaction prior to their gravitational fusion events. The Mystery of Black Hole Mergers Black holes represent some of the most powerful features of the universe. People usually visualize black holes as compact, isolated objects. Supermassive black holes exist at the center points of most massive galaxies. Such massive celestial objects exceed the weight of our Sun by a range extending from millions to billions. These enormous cosmic objects demonstrate fundamental importance for galaxy evolution; thus, we need to comprehend their behaviors to solve multiple mysteries of our universe. A gravitational wave results from the combination of merging black holes, which creates disturbances in spacetime. The LIGO observatory detected gravitational waves in 2015, which provided humans with a novel way to study the universe. Black hole mergers proceed through different stages as they release substantial amounts of energy during their rapid formation process. The black holes maintain increasing orbital distances as they approach one another before entering a tighter and tighter spiral motion. The two black holes unite in a process that produces enormous quantities of energy through gravitational wave emissions. However, there’s a problem. During the “final parsec,” the black holes remain less than a few light-years from each other yet appear to stop moving towards fusion. A vast period of stalling in the black hole merger process remains a mystery for astrophysicists to solve. Our observations of gravitational waves primarily occur when black holes are near within reach of each other. The slow final approach between black holes remains a challenge for scientists who study mergers because dark matter could fix this mystery. Dark Matter’s Role The mechanism through which dark matter supports black holes joining each other needs clarification. Overall, dark matter appears distantly related to black hole mergers only until we understand its gravitational union better. Through its gravitational powers, dark matter stands as one key mechanism that makes black holes approach one another. Dark matter prevents light absorption yet controls gravity that directs visible matter movement. The gravity of dark matter seems to accelerate black hole mergers when seen through the black hole merger scenario. Due to its active gravitational field, dark matter has the ability to attract and align black holes with each other. When black holes merge, dark matter collects in the area, which strengthens gravity enough to overcome the forces preventing the black holes from being attracted toward one another. The last part of their alignment period marks this observation. Dark matter actively works to weaken black hole energy production in addition to remaining inactive. Under the term “dynamic friction,” scientists explain this phenomenon. Astrophysicists define dynamic friction as a system where gravitational forces between matter elements reduce the motion speed of objects. Black hole mergers may experience friction effects from the possible involvement of dark matter. The black holes experience reduced orbital speed because of this force. As a result of this action, the objects draw nearer to each other. The interaction behaves like an astronomical brake system, which leads the black holes together during their ultimate merging process. The action would assist in overcoming the final parsec problem. This mechanism leads black holes to achieve the needed critical stage for gravitational waves to be generated.
How Quantum Chemistry Creates Chemical Bonds
In chemistry, we know that atoms form the chemical bonds that make up everything we use. Leading from the water we drink to the cells in our body. But there is a great science behind these connections. Quantum chemistry is a branch of chemistry. It explains how electrons behave, forming the bonds that make up our world. We will explore how electrons bond. We will also understand how they explain the science of electrons. Our focus will be on Quantum Chemistry in Chemical Bonding. What Are Chemical Bonds? Chemical bonds are the forces that binds atoms to come together into molecules. Elements can work together to make all kind of matter we see around us. Scientists usually sort bonds into three main types. The type of bond we are talking about depends on how atoms deal with electrons. That is where quantum chemistry comes in. It examines the inside workings of electron behavior. This is the reason it makes each type of bond. Quantum Chemistry’s Role in Bond Formation Quantum chemistry is needed to see how atoms and electrons interact. Unlike planets orbiting the sun, quantum chemistry shows that electrons exist at points we call orbitals. These orbitals represent regions in which electrons will most likely be found and are a vital element to an explanation of bonding. Electrons in Quantum States Each electron in an atom has a specific “quantum state,” which describes its energy and location. This idea of quantum states explains why some atoms pair up while others do not. For example, two hydrogen atoms form a bond by aligning their quantum states, allowing them to share electrons. This electron pairing stabilizes both atoms, forming a stable molecule—hydrogen gas. Why Do Some Atoms Bond? Bond probability refers to how likely atoms are to come close together. It shows how close atoms tend to be when they interact… Usually, electrons naturally like to get into the lowest energy state, and it happens with a bond. The way electrons arrange themselves in order to form stable arrangements is explained by quantum mechanics. How Electrons Define Bond Types It also explains why bonds are different in nature. Covalent bonds are when atoms share electrons so that their outer electron shells are complete. One atom donates electrons to another, and the two ionic bonds give them a positive and a negative ion, which attract. Metallic bond, which is present in metals, permits the electron to move freely between atoms to become a sea of electrons that enables metals to carry electricity and come to be ductile. The following table summarizes these bond types: Bond Type Electron Movement Example Covalent Bond Electrons are shared H₂ (Hydrogen gas) Ionic Bond Electrons are transferred NaCl (Salt) Metallic Bond Electrons move freely between atoms Iron (Fe) The quantum models, such as molecular orbital theory, enable us to visualize these bonds by indicating possible locations of electrons. Especially, this model is useful to understand the complex molecules and their characteristic bonding structure. Quantum Models in Bonding We would find that useful models for bonding exist in quantum chemistry. Molecular orbital theory is a model most commonly used to describe how atomic orbitals (electron clouds surrounding an atom) combine to form a molecule. Two atoms come close enough so that their atomic orbitals merge to allow them to share or transfer an electron. Electrons shift around to bind to form molecular orbitals that stabilize the atoms being involved in this merger. Practical chemistry applications rely upon insight into bond length, bond strength, and even the specific shapes certain molecules adopt, and molecular orbital theory gives us this insight. The Global Impact of Quantum Chemistry The explanation of bonding in quantum chemistry has important applications in the real world. Here are some examples where understanding chemical bonds is important: 1. Medicine Development Drug researchers use quantum chemistry to come up with medicines that precisely join with particular molecules inside the body. The precision could potentially yield more effective treatments with fewer side effects. 2. Material Science Knowing how atoms bond helps scientists access this fundamental control to develop new materials with tailored strength and stiffness for everything from making lighter materials for vehicles to strengthening alloys for construction. 3. Electronics and Technology It’s used to help create better semiconductors, which are needed in computers, smartphones, and other digital devices. Applications of these somehow help in understanding basically everything from technology to healthcare and beyond. Conclusion Quantum chemistry shows that chemical bonds are much more than simple attractions between atoms. Here’s a brief overview of what we found. By understanding the role of quantum chemistry in bonding, we see how essential atomic interactions are to everything around us. References For anyone interested in learning more, these sources provide additional insights:
Engineered Microbes: A Game-Changer in Plastic Waste Management
Plastic pollution feels like a never-ending story. We see it piling up everywhere around us, from landfills to oceans. This is a problem that just does not go away. Recycling helps a little. However, traditional waste methods cannot handle the mountains of plastic we produce each year. However, scientists are trying a new and exciting solution. It’s called engineered microbes. These microbes can break down plastic safely and quickly. Could these microbes, which eat plastic, be our next big step toward a cleaner planet? It persists for centuries. Some of the plastics we use can take hundreds of years to decompose. We’re creating and discarding them faster than we can manage. Every year, they contribute millions of tons of plastic to land and oceans. These plastics are laced with harm and toxins. Traditional waste management, such as landfills and limited recycling, can’t keep up. Scientists are looking to these engineered microorganisms to fill the gap. How Engineered Microbes Work? Microorganisms are nature’s recyclers. They break down dead plants, food, or other natural debris. But plastics? That is a different story. Plastic is a synthetic material with strong bonds, and most microorganisms can not digest it. The scientists are teaching microorganisms to eat plastic by modifying their DNA at the genetic level. These plastic-eating microorganisms make the enzymes needed. They break down plastic into simpler and mostly natural compounds. Here is a quick look at the benefits of these engineered microorganisms compared to traditional methods: Waste Solution Pros Cons Traditional Recycling Reduces plastic buildup Limited plastic types, requires high energy Landfills Quick and cost-effective Long-term pollution, massive space requirements Engineered Microbes Eco-friendly, adaptable, efficient Currently costly, needs scaling This table shows that the engineered microbes stand out in this table. Traditional recycling is limited to specific types of plastic. However, microbes are actually capable of recycling a wide variety of plastic polymers. This makes the process more flexible and in many ways more environmentally friendly. Bringing Engineered Microbes to the World Although these microorganisms have great potential, scaling up their use is not easy. We have questions about cost, where such things would be produced, and how they can be used safely. But they also will ensure that, released outside controlled laboratories, these microorganisms don’t do any harm to natural ecosystems. They will need a lot of testing to work on a large scale, and it is still expensive. With further research, however, these obstacles can eventually be overcome. Labs around the world are already experimenting with engineered microbes to break down plastics. In Japan, researchers have identified a bacterium, Ideonella sakaiensis, that naturally digests PET (used in water bottles). They are working on making it more effective at handling large volumes of plastic waste. In Europe, biotech companies are exploring other microbes to break down different plastic types, such as polyurethane. With more investment, these efforts could soon help tackle the world’s plastic problem. As Neil deGrasse Tyson famously said, “The great thing about science is that it’s true regardless of whether you believe it or not.” Science is helping us find real solutions, even to huge challenges like plastic waste. Future of Plastic Waste Management In the future, engineered microbes could be a routine technology in waste management facilities around the globe. These microbes are not a quick fix, but part of a continuing system for treating plastic waste. Scientists are exploring how these microbes can be used beyond just plastic. They may potentially help with other kinds of waste. This research brings us closer to a cleaner, more sustainable world. Conclusion On many levels, plastic pollution is affecting our planet, and engineered microbes are a promising way forward. They are not a magic solution, but it is an exciting step that could make a difference. These microbes evolve constantly. They could make a solution that takes plastic waste out of the issue category. This waste could become a manageable part of a healthy ecosystem.
Small Modular Nuclear Reactors: How They Work and Why They Matter
Small modular nuclear reactors (SMRs) are gaining interest across the world as they move toward clean and reliable energy. SMRs will be compact and affordable. By comparison, they will be huge and represent a new form of energy production. So what makes SMRs so unique, and why should we take notice of them? Let us check it out. What Are Small Modular Nuclear Reactors? SMRs are a new type of nuclear technology, often referred to as ‘small modular nuclear’ reactors. Unlike conventional reactors, SMRs are supposed to be small, low-maintenance, and simple to set up. The reactors, which range in size from 10 to 300 megawatts, can provide power for small towns or big industries. The modular aspect reduces construction time and costs. It allows for the construction of SMRs and allows them to be assembled on site in parts. SMRs come with higher levels of reliability and enhanced safety features the energy industry has been looking for. How Do Small Modular Nuclear Reactors Work? SMRs work in the same way that conventional nuclear reactors do. However, they offer a smaller, more flexible option. Nuclear fission is used to create heat, which generates electric power. Unlike SMRs, however, SMRs are more flexible, incorporate advanced safety designs, and can be built underground to further increase safety. Here is a simple description of SMR technology: Why Small Modular Nuclear Reactors are Important Small scalable nuclear reactors (SMRs) offer various advantages. These benefits make them a top contender in the clean energy race. Here’s why SMRs are attracting the attention of energy experts: 1. Cost-Effective Building and running traditional nuclear plants is costly. However, SMRs are smaller and can be built in a factory and transported to site. This reduces construction time and overall costs, making nuclear power more accessible. 2. Flexibility SMRs can serve areas with low power needs or work with renewable sources such as wind and solar. This adaptability makes them an attractive option for places that cannot afford or do not require large power plants. 3. Enhanced Safety Safety remains a major concern with nuclear power. SMRs address this problem through advanced safety design. Some SMRs can shut down without human intervention, reducing the risk of accidents. 4. Environmental Benefits SMRs produce minimal greenhouse gas emissions, helping to reduce dependence on fossil fuels. Additionally, the reactors are designed to create less nuclear waste, addressing one of the biggest drawbacks of conventional nuclear power. SMR Projects in Real-World Many countries have recognized the potential of SMRs and are actively working on SMR projects. For example: Country SMR Project Purpose USA NuScale Power Provides clean energy to smaller communities and industrial sites. Canada Ontario Power Generation Supports Canada’s clean energy goals by supplying low-carbon electricity. UK Rolls-Royce SMR Aims to create compact reactors for both domestic use and export markets. China Ling long One Designed to power remote islands and inland areas where large plants are impractical. Challenges of Small Modular Nuclear Reactors However, SMRs do have counterparts for certain challenges. SMRs are new and need to meet a lot of national and international standards. Regulatory approval is a significant hurdle. Secondly, nuclear power still has an image issue, even if public perception is favorable. That will involve building trust, and that will mean being open about SMR safety and benefits. SMRs are less costly than conventional reactors; however, their setup expenses can be substantial. Governments need to step up and help fund these projects. Private investors also need to contribute. This support will make SMRs a viable option for global energy needs. Future of Small Modular Nuclear Reactors Small modular nuclear reactors are exciting to build. SMRs could change the idea of nuclear power if investment and technology continue. SMRs are a stable, green solution to energy demand that grows and to climate challenges that constrict. In regions where renewables cannot meet energy demands, they could become an essential part of the global energy mix. They may even provide electricity directly to the grid. Others are working on supportive policies, and many companies are investing in SMRs. If the SMRs become more widespread, they could help countries quickly achieve their clean energy goals. This progress could be made faster and more sustainably. In fact, energy experts assert that SMRs could solve the problem of an energy future that’s balanced and reliable. Conclusion Small modular nuclear reactors have a big role to play in creating a cleaner and more resilient energy future. SMRs offer a safer, more flexible, and more affordable form of nuclear power. They provide new hope for lowering emissions and supplying our energy. With the progress we make, SMRs can be the backbone of a low-carbon, sustainable energy system. They can provide the world with clean energy with minimal environmental footprint. References