The world of cell research is buzzing with groundbreaking discoveries that are reshaping medical science. Researchers are pushing boundaries, uncovering new ways to combat diseases and enhance human health. This blog explores the most exciting advances in cell research and how these innovations are leading to important medical breakthroughs. CRISPR: Rewriting Our Genetic Code Recent advances in cell research have opened doors for treatments that were once thought impossible. Scientists have developed a new technique to manipulate cells unexpectedly and understand them. This advancement promises to advance not only our knowledge of cellular processes, but also revolutionize how we approach medical treatments. A major breakthrough is the development of CRISPR technology. This gene editing tool allows scientists to make precise changes to DNA, potentially correct genetic disorders and prevent diseases before they develop. By targeting specific genes, researchers can alter cell functions, providing hope for the treatment of conditions like cystic fibrosis and muscular dystrophy. Another significant development is advances in stem cell research. Scientists are now able to generate different types of cells from stem cells, which can then be used to repair damaged tissues and organs. This technique has already shown promise in treating conditions such as Parkinson’s disease and heart disease, replacing damaged cells with healthy ones. Organics: Miniature Organs in a Dish Scientists have developed a way to grow small, three-dimensional organlike structures called organoids. These miniature versions of human organs provide invaluable insights into disease processes and drug responses.Researchers at the Albrecht Institute created intestinal organoids to study cystic fibrosis. He used these mini guts to test personalized treatments, potentially revolutionizing drug development for this genetic disorder. Stem Cell Therapies Stem cell research is advancing rapidly. These versatile cells can develop into a variety of cell types, providing potential treatments for degenerative diseases and injuries.Japanese scientists recently used induced pluripotent stem cells (iPSCs) to create sheets of heart muscle tissue. When implanted on damaged hearts in animal models, these sheets improved heart function while paving the way for new treatments for heart failure. Single Cell Sequencing Single cell sequencing techniques allow researchers to analyze the genetic material of individual cells. This powerful technology reveals incredible diversity within cell populations, providing new insights into disease progression and treatment responses.Researchers at the Broad Institute used single cell sequencing to map brain cell types in unprecedented detail. This work could lead to better understanding and treatment of neurological disorders such as Alzheimer’s and Parkinson’s. Revolutionizing Drug Development Cell research is fundamentally transforming the drug development process. Consequently, researchers are utilizing cell cultures to test new drugs with greater efficiency, thereby reducing both the time and cost involved in bringing new treatments to market. Additionally, these cell based assays enable scientists to screen potential drugs for efficacy and safety, which, in turn, minimizes the need for extensive animal testing and preliminary clinical trials. As a result, this approach significantly accelerates the development of new therapies and enhances the likelihood of successful outcomes. Cellular Reprogramming: Turning Back the Clock Cellular reprogramming techniques allow scientists to change one cell type to another. As a result, this breakthrough offers exciting possibilities for regenerative medicine and disease modeling. For example, Harvard researchers have successfully reprogrammed pancreatic cells in diabetic mice to produce insulin. As a result, this achievement brings us closer to a potential cure for type 1 diabetes, giving hope to millions of patients worldwide. Future Prospects in Cell Research These cell research innovations represent just the tip of the iceberg. As scientists continue to unravel the secrets of cellular biology, we can expect even more important discoveries that will revolutionize medicine and improve human health.The future of healthcare looks bright, thanks to the tireless efforts of cell researchers around the world. Their work promises to usher in a new era of personalized, effective treatments for a wide range of diseases.
How Diseases Affect Cell Structure and Function
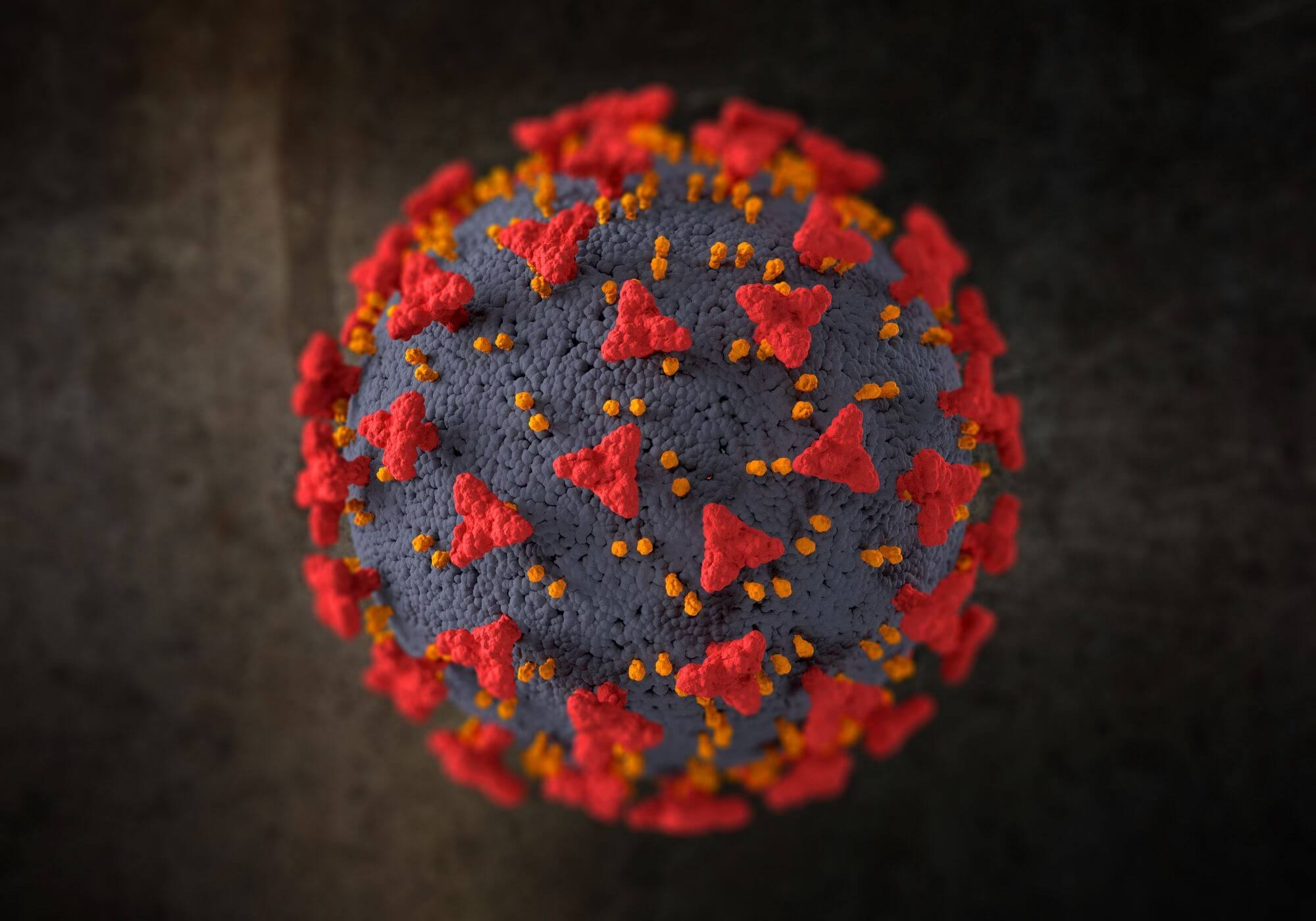
Imagine a bustling city with its streets, buildings, and infrastructure all working in harmony. Now, picture that city under attack by an invisible force that disrupts its operation. This happens to our cells when they are affected by diseases. Let’s explore how diseases can alter cell structure and functionality. By understanding these changes, we can gain insight into the complex relationship between disease and cellular health. What is Cell Structure Cells are the basic units of life; each cell has different structures that carry out specific functions. The cell membrane acts as a barrier. The nucleus holds genetic material. Organelles like mitochondria and ribosomes can execute essential functions. A healthy cell maintains a delicate balance, ensuring that all these components work together seamlessly. How Diseases Disrupt Cell Structure Diseases can wreak havoc on cell structure in several ways. For example, viruses hijack the cell’s machinery to reproduce, harm, or destroy the cell in the process. In contrast, bacterial infections can produce toxins that weaken cell membranes or disrupt organelle operation. On the other hand, cancer causes cells to grow uncontrollably, leading to abnormal cell structures and impaired performance. Viruses: The Cellular Hijackers When a virus infects a cell, it controls the cell’s machinery to replicate itself. This process often leads to significant structural changes. Cell membranes can become compromised, allowing more viruses to enter and exit the cell. Inside the cell, organelles are typically reprogrammed to aid in virus production, causing their malfunction or destruction. These changes can result in cell death and contribute to the spread of the virus within the body. The influenza virus, for example, attaches to lung cells. It then injects its genetic material inside. The normal functions of the cell stop as it becomes the virus causing factory. Eventually, the cell bursts, releasing new viruses to infect neighboring cells. Bacterial Toxins and Cell Damage Bacteria can produce toxins that directly target cell structures. For example, certain toxins can pierce the cell membrane, causing the cell to lose its integrity and die. Other toxins can disrupt the operation of the cell’s energy producers. They affect components like mitochondria. This leads to decreased energy production and cell death. These structural damages can impair tissue operation and cause symptoms of bacterial infection. Cancer and Abnormal Cell Growth Cancer signifies a unique threat to cell structure. It occurs when normal cells mutate and grow uncontrollably. These rogue cells ignore the normal growth signals of the body and avoid programmed cell death. Cancer cells often show abnormal shapes and sizes. Their internal structures become disorganized. As tumors grow, they can compress and damage surrounding healthy cells, disrupting normal tissue operation. Autoimmune diseases and cellular attack Autoimmune diseases occur when the body’s immune system mistakenly attacks its own cells. This attack can lead to various structural changes. For example, in rheumatoid arthritis, the immune system targets cells in the joints. This targeting causes inflammation and damage to cell membranes. It also impacts the underlying structures. This leads to pain, swelling, and a decrease in joint operation. These structural changes in cells can significantly lower the quality of life for individuals. People suffering from autoimmune diseases face these challenges. Neuron degenerative Diseases and Cellular Degeneration Neuron degenerative diseases, like Alzheimer’s and Parkinson’s involve the gradual loss of structure and role in neurons. In Alzheimer’s disease, abnormal protein deposits form plaques and tangles within neurons, disrupting their communication and leading to cell death. In Parkinson’s disease, the loss of dopamine producing cells impacts motor control. Structural changes in these diseases are often progressive, leading to a decline in cognitive and motor operation over time. Conclusion Diseases can have a profound impact on cell structure and operation, leading to various health problems. By understanding how these changes occur, we can better appreciate the complexity of diseases and their impact on our bodies. A virus hijacks a cell. Bacteria produce harmful toxins. Sometimes, the body’s immune system turns against itself. These changes in cell structure are critical to understanding the mechanisms of disease.
Basics of Cell Structure | The Fundamental Unit of Life
Cell structure goes beyond being a mere biological concept; it is truly the essence of life itself. In fact, every living organism, ranging from the tiniest bacteria to the largest mammals, relies on cells to function and thrive. So, what exactly comprises a cell? Let’s explore the fundamentals of cell structure, as we highlight the essential units that keep life in motion. Building Blocks of Life Cells are the building blocks of all living organisms. They come in two main types: prokaryotic and eukaryotic. Prokaryotic cells are simple and found in organisms such as bacteria. Eukaryotic cells, which include animal and plant cells, have a more complex structure. Prokaryotic Cells: Eukaryotic Cells: Understanding these cell types is important because they form the basis of how organisms’ function. Cell Membrane Every cell features a cell membrane that acts as a protective barrier. This membrane not only holds the cell’s components in place but also regulates the movement of substances in and out of the cell. Due to its selective permeability, the membrane allows only specific substances to pass through. The structure of the cell membrane consists of a phospholipid bilayer with embedded proteins, which facilitate transport and communication. Nucleus The nucleus is often described as the control center of the cell. It holds the genetic material (DNA) of the cell and coordinates activities such as growth, metabolism, and reproduction. The nuclear envelope, a double membrane, surrounds the nucleus and protects its contents. Within the nucleus, nucleolus is responsible for the production of ribosomes, which are important for protein synthesis. Cytoplasm Cytoplasm is the jelly like substance within the cell membrane, except for the nucleus. It consists of the cytosol (fluid part) and various organelles. Organelles are specialized structures within the cytoplasm, each with specific functions. For example: Mitochondria Mitochondria, often referred to as the powerhouses of the cell, play a significant role in generating energy. By conducting cellular respiration, they convert nutrients into usable energy, which is essential for various cellular functions and overall vitality. Endoplasmic reticulum (ER) The endoplasmic reticulum (ER) comes in two types: the rough ER, which lacks ribosomes, and the smooth ER, which lacks ribosomes. As a result, rough ER is mainly involved in protein synthesis while smooth ER plays an essential role in lipid synthesis. Thus, both types of ER’s are essential for the production and processing of proteins and lipids within the cell. Golgi apparatus The Golgi apparatus plays an important role not only by modifying but also in sorting and packaging proteins and lipids. As a result, it prepares these molecules either for secretion outside the cell or for distribution to different organisms within the cell. Ribosomes Ribosomes are small, dense structures found throughout the cytoplasm and on the rough ER. These molecular machines are responsible for protein synthesis, translating the genetic code into functional proteins that carry out various cellular functions. Lysosomes Lysosomes are membrane bound organelles that contain digestive enzymes. These tiny sacs break down cellular waste, damaged organisms and ingested particles. Lysosomes play an essential role in cellular maintenance and defense against pathogens. Cytoskeleton The cytoskeleton is a network of protein fibers that provide structural support to the cell. It helps maintain the shape of the cell, enables movement, and organelles within the cell. The main components of the cytoskeleton are microfilaments, intermediate filaments, and microtubules. Each component plays a different role in cellular mechanics and organization. Plant Cells Plant cells have some unique features that are not found in animal cells, such as: Conclusion Understanding the basics of cell structure provides a solid foundation for understanding biology. By exploring the different components and their functions, we gain insight into how cells work and sustain life. Every aspect, from the cell membrane to the cytoskeleton, plays an important role in the overall function of the cell. Embracing this wisdom helps us appreciate the complexity and elegance of life at the cellular level.
Dark Matter and Its Crucial Role in Galaxy Formation
Dark matter constitutes about 85% of matter in the universe, essential for galaxy formation due to its gravitational forces. It attracts ordinary matter, leading to the creation of galaxies. Evidence from galaxy rotation, cosmic microwave background, and large-scale structures supports its role. Ongoing research aims to unveil dark matter’s mysteries and influence.