Metal Organic Frameworks, or MOFs for short, have quickly become one of the most exciting materials of our time. In 2025, they secured their place in history by winning the Nobel Prize in Chemistry. This recognition goes far beyond academic achievement, it shows a global acknowledgment of a material with the power to reshape how we approach climate technology, clean energy solutions, and even access to clean water.
Imagine that you are standing in the middle of a desert, thirsty and desperate for water. Instead of searching a well, you simply use a material that can pull moisture straight from the air, like a super-smart sponge at work. It might sound like something out of science fiction, but this is exactly what MOFs can do. These tiny structures are reshaping how we capture gases, clean energy storage, and even deliver medicine inside the human body.
By the time you finish reading, you’ll clearly understand what metal-organic frameworks are, how they work at a molecular level, and why scientists say they have almost ‘superpower-like’ abilities. You’ll also see why this discovery matters for our future. We’ll keep the science easy to follow, simple, but never shallow.
What Exactly is a Metal Organic Framework?
Metal Organic Frameworks are made by linking metal clusters and organic molecules together, forming a highly porous structure. This creates tiny holes throughout the material, allowing it to trap and store gases or liquids with ease.
The Microscopic Sponge Analogy
Think of it like building a fence. You push metal posts into the ground, that’s your metal ions. Then you connect those posts with wooden planks, those are the organic linkers. Now imagine that this fence doesn’t just spread across a flat field. Instead, it grows in every direction, forming a 3D network with plenty of gaps inside.
These gaps act like tiny pores, and that’s where MOFs do their magic. They can trap gases like CO₂ or store light fuels such as hydrogen inside those spaces.
Unlike regular solid materials, MOFs aren’t heavy or tightly packed. They’re incredibly light and full of tiny holes, which allows them to hold nearly 10 times more gas than typical adsorbent materials of the same size. That’s why scientists love to call them “designer sponges at the molecular scale.”
Basic Components: Nodes and Linkers
The structure of a MOF is based on two fundamental, repeating components:
- First are the Nodes (Metal Clusters). Think of them as the corners or connection points in a Lego build. These nodes are small clusters of metal ions such as zinc, copper, or iron. They act like firm anchor points that hold everything together.
- Then come the Linkers (Organic Molecules). These work like the rods or bridges that connect one node to another. Made of organic molecules called ligands, they decide how large the gaps or pores in the structure will be.
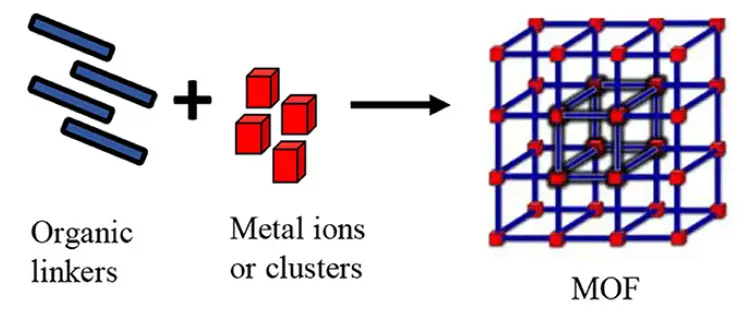
When these metal nodes and organic linkers come together through coordination chemistry, they naturally organize themselves into a highly ordered, spacious 3D framework, almost like a perfectly arranged scaffold at the molecular level.
The Simple Chemistry Behind Metal Organic Frameworks
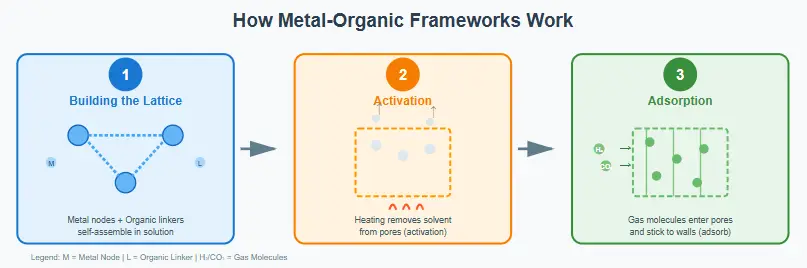
Understanding how MOFs work requires just three simple steps. Let’s break down the process.
Step 1: Building the Lattice
In this synthesis stage, we mix the metal salt and the organic ligand in a solvent and let them self-assemble. This step usually takes place under high heat, much like how crystals naturally grow. The crystal that forms has a highly porous structure, but at this point, those pores are still filled with the solvent used during the process.
Step 2: Activation – Opening the Pores
Before a MOF can start working, it needs to be activated. In this step, the solvent molecules trapped inside its structure are removed. Scientists usually do this by heating the MOF while keeping it under a vacuum. As the temperature rises, the solvent molecules escape through the pore channels.
Even after this, the strong bonds between the metal nodes and organic linkers stay firmly in place, so the structure doesn’t collapse. Instead, it turns into a clean, dry, and stable sponge-like framework. This is the point where the pores are fully open, and the material becomes ready to perform its actual function.
Step 3: Gas or Water Enters Pores → Adsorption
The main working principle here is adsorption.
Adsorption happens on the surface of a material. Molecules like gas or water vapor come into contact with a surface and stick to it, just like how tape holds things only on its sticky side.
On the other hand, absorption means a substance fully soaks something into its whole body, like a sponge pulling water into every part of it.
A MOF works through adsorption. When target molecules, such as CO₂, enter its complex network of tiny pores, they attach themselves to the inner surfaces. They stay there because of weak molecular forces, and these forces can be adjusted by changing the chemical groups present on the MOF’s organic linkers, allowing for selectivity.
A quick visual to understand surface area:
Imagine you paint a sugar cube with just one thin layer of paint. You’d only cover around 6 cm² of surface area. But just 1 gram of a high-performance MOF can hide an internal surface area equal to 10,000 to 70,000 square feet. That’s possible because MOFs fold an enormous amount of surface area into a tiny, crystalline structure. That’s why even a small vial of MOF powder can hold a surprising amount of gas.
Top 5 Superpowers of Metal Organic Frameworks
Now comes the exciting part. What can these molecular sponges actually do? Let’s explore five real applications that show why MOFs won the Nobel Prize.
1. Water Harvesting in Deserts
In areas where water is extremely scarce, MOFs can pull moisture directly from the air, even when the climate is dry. These special MOF structures soak up water vapor during the night, when the air holds a bit more humidity. When the sun rises, the heat triggers the MOFs to release that stored moisture as clean water. With this simple cycle of absorbing and releasing, MOF-based water harvesting could offer a reliable source of drinking water to communities that have no access to traditional water supplies.
2. CO₂ Capture and Climate Tech
Metal Organic Frameworks can selectively grab carbon dioxide with impressive accuracy. They pull CO₂ out of industrial emissions and can even capture it directly from the air. Due to their huge surface area and adjustable pore size, some MOFs trap CO₂ far more effectively than older techniques. This eco-friendly approach has the potential to become a powerful tool in the fight against climate change.
3. Hydrogen Storage and Clean Fuel
The hydrogen economy can only grow if we have safe and efficient ways to store hydrogen. Storing hydrogen gas is tricky because its molecules are extremely small and light, which makes them hard to contain. That’s where MOFs help. They hold hydrogen inside their tiny pores even at relatively low pressure, making storage much safer and more practical for fuel cell vehicles and renewable energy systems.
4. Drug Delivery and Biomedical Use
Imagine a medication that doesn’t rush through your body all at once, but instead releases slowly, exactly where it’s needed. That’s what scientists are working on with biocompatible MOFs. These tiny structures act like molecular cages, safely holding drug molecules inside their pores. They protect the medicine during its journey through the body and only release it once it reaches the right spot, at a steady, controlled pace.
5. Chemical Sensing and Filtration
MOFs work like smart filters, when certain molecules enter their tiny pores, they respond by changing color or shifting their electrical properties. Because of this reaction, we can use them to monitor the environment, improve safety in industries, and even detect explosives. What makes them especially useful is their ability to recognize and separate specific contaminants while allowing other harmless molecules to pass through.
Why Metal Organic Frameworks Just Won a Nobel Prize
The 2025 Nobel Prize in Chemistry honored the scientists who pushed metal organic frameworks to a whole new level. Their work didn’t appear overnight, it grew from years of progress in coordination chemistry. But the Nobel Committee made it clear why this moment stood out: MOFs didn’t just add to existing knowledge; they opened a completely new direction in materials science.
The real breakthrough wasn’t simply making these structures. It was proving that MOFs could be designed on purpose. Scientists showed that these materials aren’t lucky discoveries. They’re programmable. If you change the building blocks, you can change the behavior and properties of the material. That level of control is what makes MOFs game-changing.
To put it in everyday words: this research could help us pull clean water from dry air in drought-hit regions or store clean fuel for zero-emission vehicles. MOFs are more than a scientific achievement, they’re a toolbox for solving real-world problems that impact billions. This recognition proves how deep chemistry research can lead directly to the technologies our future will rely on.
Limitations Nobody Explains Clearly
MOFs sound like magic materials, but let’s talk honestly about the challenges. Science education should include both promises and limitations.
1. Cost and Scalability
Making high-performance MOFs isn’t cheap. The raw materials alone can cost a lot, and the process usually needs special solvents and very controlled temperatures. In the lab, producing a few grams is manageable, but scaling that up to metric tons for real-world industrial use becomes a serious engineering and financial challenge. Green chemistry is working toward finding affordable and eco-friendly ways to make MOFs, but for now, the high production cost still limits their widespread use.
2. Stability and Regeneration Energy
For a MOF to actually be useful in real-world applications, it has to keep working for thousands of cycles without breaking down. It should stay chemically and thermally stable even when exposed to humidity, pollutants, or high temperatures. When it’s time to release the trapped molecule—like CO₂—the MOF needs to go through a “regeneration” process, which usually involves applying heat or creating a vacuum. But here’s the catch: the energy needed for regeneration must be lower than the benefit the MOF provides. Otherwise, the entire process becomes impractical and not worth using.
To keep it real, MOFs are a brilliant innovation, but moving from laboratory success to real-world impact is a big challenge. We need cost-effective MOF materials that stay stable and use very little energy during regeneration. The Nobel Prize highlights their potential, but the real test is making them scalable and efficient for everyday use.
Summary of Key Points
| Term | Simplification |
|---|---|
| Adsorption | Molecules sticking to a surface, like tape (the MOF function). |
| Absorption | Soaking up a substance into the volume, like a sponge. |
| Ligand | The organic molecule that connects the metal nodes (the linker molecule). |
| Porosity | The measure of empty space or holes within the material (the key to MOF power). |
| Activation | The process of heating a MOF under a vacuum to remove solvent and open the pores. |
| Surface Area | The total internal area available for adsorption within the MOF’s pores. |
| Coordination Bond | The chemical bond that locks the metal node and the organic linker together to form the lattice. |
Conclusion
At Learning Breeze, we don’t see chemistry as a list of formulas to memorize. We see it as something alive, something that connects directly to real-world solutions.
Metal organic frameworks are a perfect example. They began as curious lab experiments. Today, they stand as breakthrough materials with the power to capture carbon from the air, harvest water from dry desert air, and store clean hydrogen fuel. That’s not just chemistry, that’s future technology being built right now.
When you understand MOFs, you see science differently. It’s no longer just equations on paper. It becomes a creative process, designing molecules that solve human faced challenges. Whether you’re studying for exams or just curious about how science influences the future, this understanding matters. The materials we create today will shape what’s possible tomorrow, and now you know one of the most exciting innovations of our time. Want to test your understanding? Try our Organic Chemistry Quiz and see how much you really know, challenge yourself!
Recommended Reads for Curious Minds
If you want to explore more about porous materials, coordination chemistry, and how they’re used in industries, here are some helpful resources we recommend.
- Handbook of Porous Materials: Synthesis, Properties, Modeling and Key Applications by Editor-in-chief: Vitaly Gitis
- Introduction to Materials Science for Engineers by James F. Shackelford
- Introduction to Reticular Chemistry: Metal-Organic Frameworks and Covalent Organic Frameworks by Omar M. Yaghi
FAQs About Metal Organic Frameworks
MOF stands for Metal Organic Framework. It describes a type of porous material created when metal ions or clusters join with organic linkers. Together, they form a solid, crystal-like structure that looks organized at the molecular level.
The main purpose of MOFs is to adsorb, store, or separate molecules. They are widely researched for carbon capture, gas storage, drug delivery, catalysis, and water purification due to their customizable structure and high porosity.
MOFs have a repeating 3D lattice structure, resembling a sponge at the microscopic level. They contain millions of tiny pores that can trap or release molecules, giving them extremely high surface area—often higher than activated carbon or zeolites.
A well-known example of a MOF is MOF-5 (also called IRMOF-1), made from zinc ions and terephthalic acid linkers. It is widely studied for hydrogen storage and gas separation due to its stable and highly porous structure.
References
- MacGillivray, L. R. (Ed.). (2010). Metal Organic Frameworks: Design and Application. Wiley.
- Kaskel, S. (Ed.). (2016). The Chemistry of Metal-Organic Frameworks, 2 Volume Set. Wiley-VCH.
- Yaghi, O. M., Kalmutzki, M. J., & Diercks, C. S. (2019). Introduction to Reticular Chemistry: Metal-Organic Frameworks and Covalent Organic Frameworks. Wiley-VCH.
- Glover, T. G., & Mu, B. (Eds.). (2018). Gas Adsorption in Metal-Organic Frameworks: Fundamentals and Applications. CRC Press.
- Furukawa, H., Cordova, K. E., O’Keeffe, M., & Yaghi, O. M. (2013). The chemistry and applications of metal-organic frameworks. Science, 341(6149), 1230444.
- Zhou, H.-C., Long, J. R., & Yaghi, O. M. (2012). Introduction to metal-organic frameworks. Chemical Reviews, 112(2), 673–674.
- Kim, H., Yang, S., Rao, S. R., Narayanan, S., Kapustin, E. A., Furukawa, H., … & Yaghi, O. M. (2017). Water harvesting from air with metal-organic frameworks. Science, 356(6336), 430–434.
- Sumida, K., Rogow, D. L., Mason, J. A., McDonald, T. M., Bloch, E. D., Herm, Z. R., … & Long, J. R. (2012). Carbon dioxide capture in metal-organic frameworks. Chemical Reviews, 112(2), 724–781.
- Murray, L. J., Dinca, M., & Long, J. R. (2009). Hydrogen storage in metal-organic frameworks. Chemical Society Reviews, 38(5), 1294–1314.
- Horcajada, P., Gref, R., Baati, T., Allan, P. K., Maurin, G., Couvreur, P., … & Serre, C. (2012). Metal-organic frameworks in biomedicine. Chemical Reviews, 112(2), 1232–1268.
- Nobel Prize Committee. (2025). Press release: The Nobel Prize in Chemistry 2025. NobelPrize.org.
- Li, J.-R., Sculley, J., & Zhou, H.-C. (2012). Metal-organic frameworks for separations. Chemical Reviews, 112(2), 869–932.




2 Comments
Most interesting. Thank you for making this info available. Brings hope for the long term.
Glad it sparked hope, thanks for reading and sharing the positive vibe!