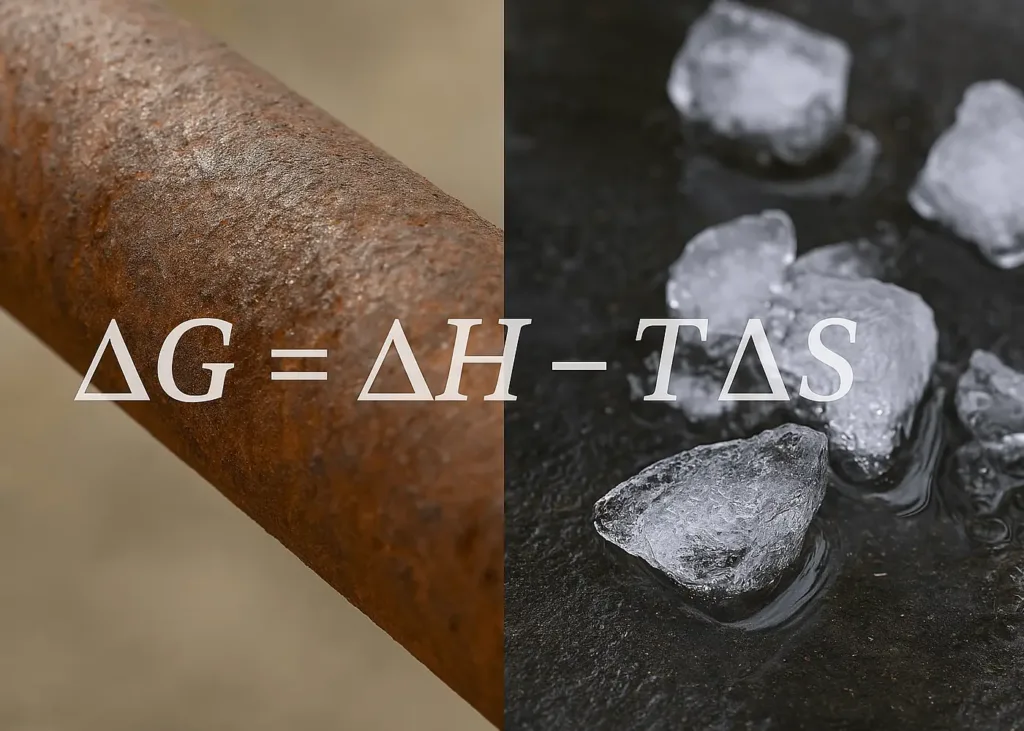
Have you ever thought about why some things just happen, like ice melting on a sweltering day or iron turning all rusty over time, while other stuff needs a little nudge to get going? What is the deal with that? Well, it all comes down to this cool concept called Gibbs free energy.
In this guide, we are going to make sense of Gibbs free energy without all the technical jargon. We’ll keep it simple. You will find what it actually is, why it’s important, and how to use its formula to figure out if certain chemical reactions will just happen naturally or if they need a bit of help.
By the time you finish reading, you will have a solid understanding of how to calculate Gibbs free energy. You will see how it ties into everyday stuff like how we produce energy and the reactions happening in living things. Let’s dive in!
What is Gibbs Free Energy?
Gibbs free energy works as nature’s scorekeeper. It signifies the “useful energy available to do work” in any chemical or physical process. Just like how a ball rolls downhill due to gravity. Chemical reactions tend to move towards the state of lower Gibbs free energy.
To really get a grip on this concept, picture your messy bedroom. You know how it seems to drift towards chaos? That’s entropy at work. Meanwhile, you want to keep your energy use low-think of that as enthalpy. Gibbs free energy is like the mediator between these two opposing forces. It helps us find out if a process will happen on its own by comparing changes in energy to changes in disorder.
This whole idea was given by Josiah Willard Gibbs, a brilliant American scientist who introduced it in the 1870s. His insights changed the game for how we look at chemical reactions and energy transfer. Nowadays, holding Gibbs free energy is very important for predicting whether reactions will happen spontaneously, whether we are talking about biological processes or even industrial manufacturing.
Although Gibbs free energy has this amazing predictive ability. Instead of shelling out big bucks for experiments just to see if a reaction will take place, scientists can crunch the numbers with Gibbs free energy changes. They can quickly tell if the process is thermodynamically favorable or not.
How Gibbs Free Energy Determines Spontaneity
Alright, let’s take a closer look at Gibbs Free Energy and how it helps us understand whether reactions happen on their own. But before we get into that, we need to clarify what “spontaneous” means in thermodynamics.
What Does Spontaneous Mean?
When scientists say something is “spontaneous,” they are essentially saying it can just happen by itself, without needing any extra energy. For example, diamonds. They can slowly transform into graphite over millions of years—that’s spontaneous. But then there’s dynamite, which can explode spontaneously, in just a blink of an eye, like a few milliseconds.
You can think of spontaneity like water flowing down a hill. It just flows naturally from higher ground to lower ground unless something gets in the way. Similarly, spontaneous reactions will occur if the conditions are right and there’s enough time, even if there are some hurdles in the path. That’s a whole different ball game called kinetics.
The Role of ΔG
So, when it comes to figuring out if a reaction will happen on its own, the change in Gibbs free energy (ΔG), is very important. Let’s break it down in a straightforward way:
| ΔG Value | Process Type | What It Means |
|---|---|---|
| ΔG < 0 (negative) | Spontaneous | Process can occur naturally |
| ΔG > 0 (positive) | Non-spontaneous | Process requires energy input |
| ΔG = 0 | Equilibrium | Process is balanced, no net change |
When ΔG is negative, it releases free energy that can carry out useful work. When ΔG is positive, you must supply energy to make the process happen. At equilibrium (ΔG = 0), the onward and reverse processes balance perfectly.
This simple rule applies universally, from the smallest molecular interactions to large-scale industrial processes. It’s one of the most powerful tools in chemical reactions.
How to Calculate Gibbs Free Energy
The Formula: ΔG = ΔH – TΔS
The Gibbs Free Energy formula might look intimidating at first, but each part has a clear meaning:
- ΔG = Change in Gibbs Free Energy (what we want to find)
- ΔH = Change in enthalpy (heat absorbed or released)
- T = Temperature in Kelvin (absolute temperature)
- ΔS = Change in entropy (disorder change)
Let’s break this down a bit. Imagine this equation like a tug-of-war between two players: enthalpy and entropy. Enthalpy (ΔH) is all about the heat energy change, while entropy, noted as ΔS, deals with how much disorder there is. And then there is temperature, T, which sort of cranks up the impact of entropy.
When we are talking about standard conditions—like 25°C and 1 atmosphere—scientists use specific values marked with a little superscript °. This helps everyone around the world compare their findings consistently.
Now, here’s where it gets interesting: that negative sign in front of TΔS is very important. It tells us that when disorder increases (you know, that positive ΔS), it actually makes ΔG more negative. And why does that matter? Because it makes things more spontaneous, which totally fits with how nature seems to lean towards more disorder.
A Simple Example
Let’s calculate the Gibbs Free Energy for melting ice at 298 K (25°C):
Given data:
- ΔH = +6.01 kJ/mol (energy needed to break ice structure)
- ΔS = +22.0 J/mol·K (liquid water is more disordered than ice)
- T = 298 K
Step-by-step calculation:
- Convert ΔS to the same units as ΔH: 22.0 J/mol·K = 0.022 kJ/mol·K
- Apply the formula: ΔG = ΔH – TΔS
- Substitute values: ΔG = 6.01 – (298 × 0.022)
- Calculate: ΔG = 6.01 – 6.56 = -0.55 kJ/mol
Since ΔG is negative, ice melting at 25°C is spontaneous. This is akin to our everyday experience—ice cubes melt in room temperature water without any external energy contribution.
Why Gibbs Free Energy is Important in Everyday Life
Gibbs Free Energy isn’t just some dry concept from your chemistry textbook—it actually plays a huge role in shaping the world we live in.
Let’s break it down a bit:
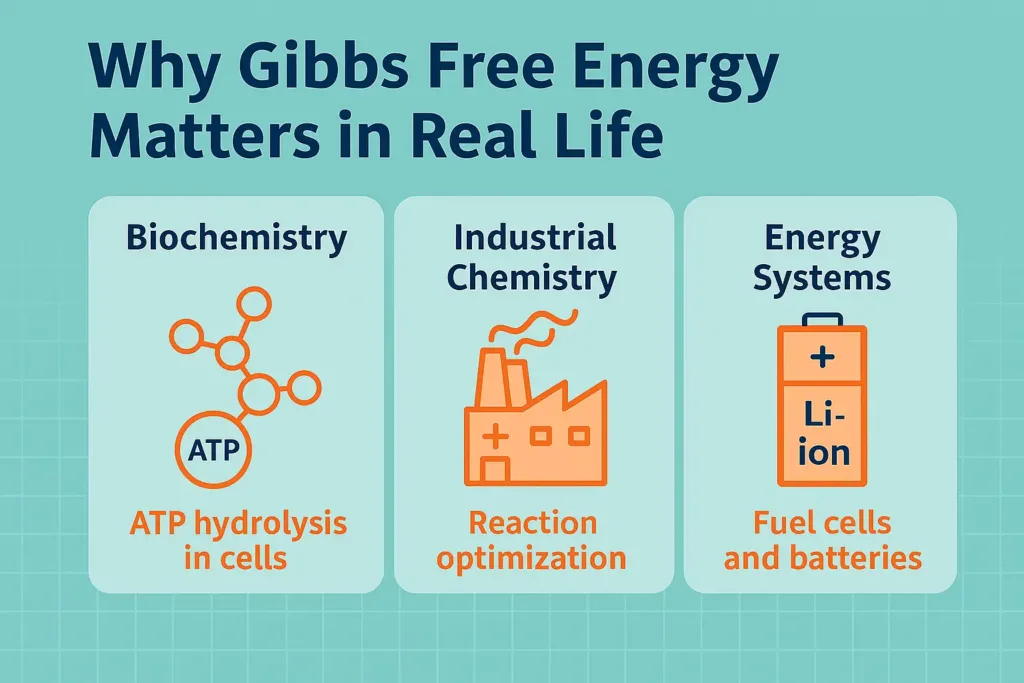
- Biochemistry: Take a look at what’s happening in your body. When ATP gets hydrolyzed, it’s got a negative Gibbs Free Energy (ΔG). This means it releases energy, which is crucial for all sorts of actions—like when your muscles contract or when your nerves send signals.
- Industrial Chemistry: Now, in the industrial world, Gibbs Free Energy helps manufacturers choose which reactions to pursue. They focus on spontaneous reactions—those that happen naturally—because it cuts down on energy costs, especially in processes like making ammonia.
- Energy Systems: And then there are fuel cells and batteries. These rely on spontaneous electrochemical reactions too. When there’s a negative ΔG, it leads to efficient energy production. If you peek at a diagram of a fuel cell, you’ll see how these spontaneous reactions are what power electricity generation. It’s fascinating to think about how Gibbs Free Energy is a key player in developing sustainable energy solutions.
So, next time you hear about Gibbs Free Energy, remember—it’s not just science jargon; it’s part of the energy mystery that keeps our world running.
Common Misconceptions & FAQs
Misconception 1: Gibbs Free Energy is ‘Free’ to Use
The term “free” can really throw people off when they first face it. When we talk about Gibbs free energy, it’s not “free” like you might think in terms of money. It’s a bit different. Here, “free” means it’s the energy that’s actually available to do some useful work, but only under specific conditions, like constant temperature and pressure.
Just for a moment, consider your financial situation. You have money sitting in your checking account that you can spend whenever you want—that’s your “free” cash. On the other side, if your money is tied up in investments, it’s not quite as accessible for immediate use. So, in the same way, Gibbs free energy is all about that slice of total energy that is right there, ready to be used for useful work.
Misconception 2: Spontaneous Means Fast
This is a very common misunderstanding. A lot of people think spontaneous processes happen quickly, but that’s not always the case. Take diamond formation, for example, once again. It is spontaneous under the right conditions, but we’re talking about timeframes that stretch over millions of years—geological time scales.
On the other side, there are non-spontaneous processes that can move along very quickly if you pump in enough energy. So, it’s important to remember: spontaneity is all about whether something can happen at all, while kinetics is about how fast it actually goes down. They are two different things in chemistry and physics, and mixing them up can lead to some real confusion.
Frequently Asked Questions:
- Can (ΔG) be negative? Yes, indicating a spontaneous process.
- How does temperature affect (ΔG)? Higher temperatures amplify entropy’s effect, potentially making (ΔG) more negative.
- Is Gibbs Free Energy the same as Helmholtz Free Energy? No, Helmholtz Free Energy ((A = U – TS)) is for constant volume systems.
Conclusion
Gibbs free energy is somewhat fascinating when you think about it. It’s like nature’s way of telling us whether things will just happen on their own. There is this straightforward equation: ΔG = ΔH – TΔS. It is a neat little formula that connects changes in energy to changes in disorder, giving us some solid insights into how both chemical and physical processes work.
This concept opens up a whole new world once you get your mind around it. I mean, it’s relevant to everything from how our cells work to big industrial processes. Whether you are diving into biochemistry, studying chemical engineering, or just curious about how things happen in the world, Gibbs free energy is crucial. It helps in understanding both energy and spontaneity. With this knowledge under your belt, you are all set to handle some more complex topics, like chemical kinetics, thermodynamic equilibrium, or even statistical mechanics.