When we think about quantum physics, Heisenberg’s uncertainty principle often comes up. This principle has been the basis of quantum mechanics, expressing that we can’t accurately measure both the position and momentum of a particle at the same time. For decades, this principle has been widely accepted. However, some scientists are beginning to challenge this unshakeable principle. What is giving rise to this skepticism? Could it be that Heisenberg’s principle, once a basic principle of modern physics, may not be as solid as we thought? We will talk about these interesting challenges. We will explain in detail how they emerged and what they could mean for the future of science. Get ready for a journey into this surprising turn of events! Heisenberg’s Uncertainty Principle Heisenberg’s uncertainty principle revolutionized our understanding of the quantum world. It states that there is a limit to how precisely we can know both the position and momentum of a particle. The more accurately we measure one, the less precisely we can measure the other. This principle fundamentally is based on the wave-particle duality of matter. Here are the core ideas: These points have traditionally supported the theory; however, new studies are challenging these assumptions. Recent Developments Recent research suggests that our traditional interpretation of the uncertainty principle may be outdated. Here is a summary of the most common theories and insights of scientists who are questioning: 1. Quantum Measurements New techniques allow scientists to measure quantum states with greater accuracy. Researchers are experimenting with methods that could potentially refine or challenge the limits set by the Heisenberg’s principle. 2. Quantum Entanglement Some researchers believe that particles can be entangled in a way that would allow precise measurements of both their position and momentum. 3. Experimental Results Some experiments have observed phenomena that seem to conflict with the traditional perception of uncertainty. These observations may suggest that our understanding of quantum mechanics is incomplete. 4. Theoretical Revisions Physicists are looking at the mathematical foundations of quantum mechanics again. There is ongoing debate over whether the uncertainty principle is a fundamental limit or an approximation. Experiments Challenging the Theory A series of experiments have given rise to discussions about the validity of Heisenberg’s uncertainty principle. Here are some notable experiments. 1. Danish Experiment The Danish researchers used advanced laser technology to measure the particles, without causing as much damage as traditional methods. 2. Cavity Quantum Electrodynamics This experiment showed that information about both position and momentum could be obtained with less interference than formerly thought. The results of these studies could be very significant, encouraging the physicists to reconsider the basic principles of quantum mechanics. Implications for Quantum Physics Some of the biggest names in physics are adding their voices to this debate. For example, theoretical physicist Sabine Hossenfelder has argued that the uncertainty principle may be less fundamental than earlier thought. She suggests that as our understanding of quantum mechanics grows, it may eventually be replaced by a more precise theory. Similarly, Nobel laureate Gerard ‘t Hooft has pointed out that although the principle works well for most quantum systems, there may be special cases where it doesn’t work. This idea has inspired ongoing research into the nature of particles, waves, and the limits of quantum theory. Will we ever replace Heisenberg’s uncertainty principle? It is too early to say for sure. Most physicists agree that the principle will stay important for a long time. However, as more evidence develops, it is possible that it could be revised or even replaced by a more thorough theory. It is clear that the principle is no longer unbreakable. Physicists are willing to reconsider old ideas, and Heisenberg’s uncertainty principle is no exception to this process. Whether these are minor or major changes, the future of quantum mechanics could look quite different. Conclusion As scientists continue to put doubt on Heisenberg’s Uncertainty Principle, we find ourselves at the beginning of a new era of quantum understanding. While this principle serves as a fundamental principle in physics, questioning its absolute validity opens up exciting new fields of scientific study. The questions raised by recent research force us to reexamine not only the principles of quantum mechanics, but also the technologies that come from them. In conclusion, as the journey through quantum physics evolves, perhaps we are on the verge of understanding not only “what” exists at the particle levels, but also “how” and “why” those particles behave the way they do. As we stand at this crossroads of inquiry and innovation, the future of physics looks bright, even if it is uncertain.
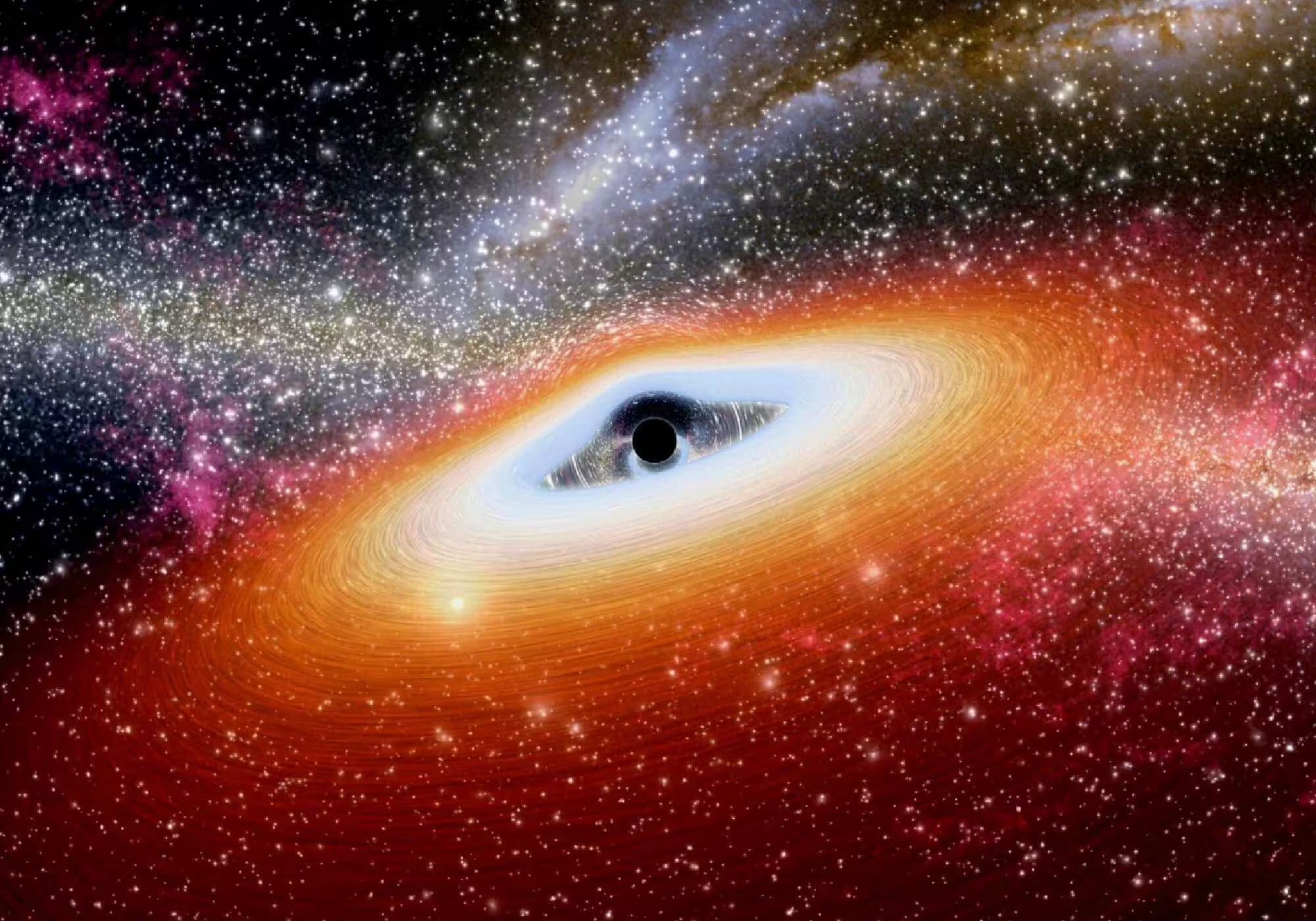
Unveiling TON 618: The Largest Known Black Hole and Its Mind-Blowing Secrets
TON 618 is the largest known black hole in the universe, discovered in the 1970s through quasar observations. Its immense size and gravitational pull play a crucial role in galaxy formation, impacting the movement of stars and gas. Research on TON 618 enhances our understanding of black holes and the universe’s limits.
Green Chemistry is Transforming Sustainable Innovation
Green chemistry is making a positive impact across industries. Changing the way we make products that are safer for the environment and ourselves. You have probably heard of it before, but what does it mean for everyday life and sustainable innovation? Green chemistry is now a reality in industries around the world. From the cleaning products we use in our homes to the materials used in today’s innovative technologies, the principles of green chemistry are sparking a revolution. In this blog, I’m going to share how green chemistry is taking sustainability to new heights, transforming industries, and helping us build a greener, healthier planet. Power of Green Chemistry Sustainable chemical processes focus on designing processes and products that reduce waste, minimize the energy use, and limit harmful substances. This approach benefits both the environment and human health by reducing the exposure to toxic chemicals. As businesses try to fight climate change, they are including green chemistry in their plans. How Green Chemistry is Transforming Sustainable Innovation 1. Reducing Waste and Pollution Green chemistry is known for its ability to reduce waste. Traditional manufacturing processes often generate tons of waste, and a lot of which is harmful for human health. This method tackles the problem by developing processes that use materials more efficiently, and leaving fewer harmful byproducts behind. For example, in the pharmaceutical industry, green chemical processes have reduced hazardous waste by 90%. This makes a huge difference. 2. Safer Products These new practices also focus on making products more environmentally friendly. From biodegradable plastics to nontoxic cleaning agents, companies are developing products that perform well without harming the human health. These products typically come with labels such as “nontoxic” and “biodegradable,” to indicate their greener, safer nature. This means a lower risk for consumers and a safer environment. 3. Energy Consumption Energy use has long been a challenge for industries, but sustainable chemical methods offer solutions. New chemical processes now take place at lower temperatures and pressures, significantly reducing the use of energy. By reducing energy costs and carbon footprints, companies are saving money and reducing their impact on the planet. Every step counts when it comes to sustainability. 4. Renewable Materials These methods have opened the door to renewable materials. Instead of relying on petroleum based plastics, it is now possible to produce bioplastics from renewable sources such as corn or sugar cane. These bioplastics are not only sustainable, but also break down more easily, creating less long term waste. This is the future of materials. Role of Green Chemistry in Innovation Sustainable chemistry is not just about making changes to existing products or processes, it’s about rethinking how we engage with chemistry. Green chemistry encourages sustainable practices from the start, leading to innovations that impact entire industries. It opens the door to creative solutions that prioritize the environment without compromising quality or performance. Many companies are moving in this direction. For example, Patagonia uses environmentally friendly materials, while the Method is creating safe, biodegradable household items. These brands are setting a trend, proving that green chemistry is not just the future, it is the present. Future for Green Chemistry The future of these practices looks very bright. With continued research and advancements, sustainable practices will become more accessible to industries everywhere. We may soon see all plastic packaging easily biodegrade, energy intensive processes replaced by low energy sources of energy, and chemical waste become a thing of the past. It’s an exciting time for innovation. Green Chemistry Matters Now Sustainable chemical methods are essential, with climate change and environmental concerns being more urgent than ever. It offers businesses and consumers real solutions to make a positive impact on the planet. As more industries begin to use these methods, we will see significant steps towards a sustainable world. Benefits of Green Chemistry Conclusion Green chemistry is changing the competitive environment. By reducing waste, minimizing energy use, and creating safer products, it is contributing to sustainable innovation across industries. As we implement these practices, the future looks bright and green. And for someone like me, who is passionate about science and sustainability, it is thrilling to see this change happening.
Entropy in Thermodynamics | Why Entropy Always Increases
Thermodynamics is full of interesting concepts, but none of the concept is as exciting as entropy. In simple words, entropy measures the amount of disorder in a system. Over the time, this disorder naturally increases, which is why you often hear phrases like “entropy always increases.” But why does this happen? Today, we will explain how entropy works in thermodynamics and why it’s so important to understand. What is Entropy? Firstly, we need to understand the basics of entropy. In thermodynamics, entropy is defined as the amount of randomness or instability in a system. When we observe any natural process, whether it is ice melting into water or a car engine heating up, entropy plays an essential role. Things don’t stay the same forever, they move from order to disorder, making entropy a key role in explaining why many processes are irreversible. Why Entropy Always Increases The concept of entropy has its origin in the second law of thermodynamics. This law states that the total entropy of an isolated system can never decrease over time. It either remains constant or increases, depending on the processes taking place. The reason is that: 1. Natural processes promote entropy Systems naturally continue to evolve as they reach a state of higher entropy. Imagine marbles falling into a cup. At first, the marbles are ordered in the cup, but once scattered, they become disordered. It is much easier for them to scatter than to fall back into the cup in the same order. This is a simple example of how entropy increases in our daily lives. 2. Energy Dispersion In any process, energy dissipates. A cup of hot coffee loses heat over time, and that heat dissipates into the air. As a result, the energy becomes more spread out, increasing the overall entropy. You cannot return that heat back without adding more energy to the system. 3. Irreversibility Consider the example of melting ice. When ice melts into water, molecules that were once tightly packed into a solid structure begin to move around freely. This creates more disorder (or higher entropy). Once water is melted, it is impossible to return that water to its previous state without the expenditure of energy to refreeze it. As a result, this clearly shows that how natural processes, without outside intervention, increase entropy. Daily Life Examples of Entropy To better understand how entropy in thermodynamics affects everything around us, let’s take a look at some common examples: Entropy on large scale On a larger scale, entropy helps us to explain the evolution of the universe. The universe is constantly expanding, and with this expansion, energy expands. Stars burn their fuel, planets radiate heat, and black holes emit radiation. All of these processes lead to an increase in overall disorder. This means that, as time goes on, the universe becomes less organized. The constant increase in entropy may eventually lead to a state called “heat death,” where all energy is equally distributed, and no more work can be performed. How Does Entropy Impact Us? Entropy is not just a theoretical concept; it has practical implications for everything from engineering to our daily lives. Understanding how energy spreads out can help us make more efficient engines, create better insulation, and even predict the change in the weather. In our daily activities, entropy explains why things break down over time and why it takes effort to maintain order. Whether it’s cleaning our house, organizing our files, or maintaining a healthy body, the fight against entropy is an endless process. Conclusion Entropy is a fundamental concept in thermodynamics that explains why the universe behaves the way it does. It controls how energy flows, how time goes, and how disorder grows. By understanding entropy, we can better understand the natural processes that determine our world.